- Joined
- Aug 3, 2010
- Messages
- 4,654
- Likes
- 8,364
Abstract
An iron sword from an Iron Age megalithic burial at Thelunganur in Tamil Nadu, India, was examined using metallographic techniques. The sword was made of ultra‐high‐carbon steel with a fairly uniform microstructure consisting primarily of fine cementite particles in a ferrite background free of notable non‐metallic inclusions. The morphological control, however, was not perfect and frequently allowed cementite to precipitate in the form of a network along austenite grain boundaries. It was also observed that carbide particles of varying size and shape often caused microscopic layers to develop, forming a visible pattern to the naked eye on the polished and etched surface of the iron sword. This pattern likely inspired the later development of various surface markings such as the damask. This paper presents a detailed account of the analytical data to show that the iron sword under consideration was an early example of high‐carbon steel employed in the manufacture of a functional object where the divorced eutectoid transformation technique, rediscovered recently, was used for the control of cementite morphology. It is also proposed that technologies for making and handling high‐carbon steel were in existence at a much earlier date than previously supposed.
INTRODUCTION
Carbon concentration and its distribution pattern constitute two key factors that determine the functional properties of an iron sword, which can be optimized by taking the balance between strength and ductility. The selection of raw materials therefore plays an important role in the establishment of a particular sword‐making technology. If high‐carbon steel were used to take advantage of its high strength, it would be necessary to improve the low‐impact resistance arising from its high carbon concentration.
India was famed for the early production of a special high‐carbon material termed crucible steel. In his survey research on traditional Indian crucible steel, wootz, Bronson (1986) concluded that it was produced by a wide range of processes, not only just as super steel but also as a material for common use. Despite its fame gained as the raw material for Damascus blades, Bronson noted that crucible steel was not a material of choice among warriors, primarily due to its brittleness. This brittleness arises from its high carbon concentration, which is generally > 1%. (Chemical compositions in this paper are based on weight fraction.) Such high‐carbon steel contains a substantial amount of iron carbide (Fe3C), termed cementite, which is brittle even at temperatures up to its melting point. This brittleness poses serious difficulties in fabrication or service and cannot be overcome without a high level of technological sophistication.
Raw materials similar in carbon levels to Indian crucible steel were produced elsewhere in the world using a variety of techniques, most of which involved the use of cast iron in one way or another. Such high‐carbon materials were then employed as intermediaries to be made into finished items with processes that caused a substantial reduction of their carbon level (Park 2004, 2005, 2008). Evidence was found, however, that the technology for processing high‐carbon steel in India was rather unique and focused on controlling the shape, size and distribution of the cementite phase (Park and Shinde 2013b). In a certain sense, therefore, the discovery of proper techniques for handling such high‐carbon materials signifies the beginning of crucible or other high‐carbon technologies, and Indian ironworkers should thus also be recognized for their in vention of such processing methods.
Bronson (1986) traced the beginning of Indian crucible technology to the second century ce , at the earliest, by refuting the prevailing hypotheses of earlier dates based on certain classical sources of the 5th‐century bce onwards. He also rejected Hadfield's interpretation that some of the iron objects excavated from the Sirkap site at Taxila, Pakistan, were made of crucible steel (Marshall 1945). In a subsequent review article drawing on an extended body of literary and archaeological evidence, Craddock (1998) reinforced most of Bronson's conclusions. Craddock, however, regarded the Taxila iron objects examined by Hadfield as an example of crucible steel, and suggested that crucible processes were practised by the first centuries of the first millennium ce . On the other hand, recent studies (Srinivasan 2007; Srinivasan et al . 2009), based on the investigation of crucible fragments and metal particles recovered from ancient sites in Tamil Nadu (Rajan 1997), proposed that the crucible process was likely practised as early as the mid‐first millennium bce .
Despite substantial debate over the origin and production of Indian crucible steel, little is known of thermomechanical treatments developed for the fabrication of such high‐carbon materials into finished products. Much work (Verhoeven and Peterson 1992; Verhoeven et al . 1996) has been done on Damascus swords to understand the mechanisms responsible for the emergence of their unique surface patterns. However, the information from such research cannot be used to infer technologies practised in Indian antiquity. In this respect, the recent work of Park and Shinde (2013b) is significant as it reported on a small piece of unprocessed high‐carbon steel and small finished items evidently made from such a high‐carbon material. In addition, they produced radiocarbon data placing the date of manufacture to the last few centuries of the first millennium bce . The objects these authors examined, however, were ordinary items weighing approximately 10 g, or less. Their data, therefore, cannot be extended to address the technological and chronological aspects associated with the use of high‐carbon steel in the manufacture of important functional items, such as swords. Similarly, the analysis of a Sasanian sword dating to the sixth to seventh centuries ce (Lang et al . 1998), one of the earliest examples of such products thus far examined, is also far from sufficient.
A unique opportunity to resolve this lack of information is provided by an iron sword (Fig. 1) recently recovered from an Iron Age megalithic burial site at Thelunganur in the Salem district of Tamil Nadu, India (Fig. 2). The site can be dated to the sixth century bce or earlier based on typological grounds (Rajan 2004; Rajan and Yatheeskumar 2012), indicating that the sword could be an early archaeological example of a high‐carbon material successfully processed into a finished product. The results presented below are expected to shed a new light upon the discussion of Indian high‐carbon steel in terms of its technology and chronology.

General appearance of the double‐edged iron sword under investigation: (a) general appearance, where arrows locate the spots from which specimens were taken for metallographic examination; (b, c) magnified view of the tip and the body, respectively; and (d, e) magnified views of the hilt. [Colour figure can be viewed at wileyonlinelibrary.com]
COMMENTS ON ARTEFACT AND SITE
Fig. 1(a) shows the general appearance of the sword, with the arrows indicating the spots from which tiny test specimens were taken for examination. The sword is double edged, approximately 88 cm long and 930 g in weight. The blade is around 4.7 cm wide near arrow 4 and becomes narrower in both directions toward the hilt and the tip. It is 3.7 cm wide where the hilt begins. Fig. 1(b, c) show two parallel grooves, approximately 5 mm apart, incised along the midrib. They start approximately 10 cm from the hilt and run toward the tip until they are barely visible against the corroded surface near the tip. The midrib of the blade is around 3.4 mm thick near arrow 3 and 7.7 mm thick between arrows 5 and 6. The tip near arrow 1 is ≤ 1 mm in thickness, indicating that it was subjected to significantly more plastic flow than the other parts. The hilt of the blade was presumably designed for hafting (Fig. 1, d, e), and there are two rods extending from the upper edge of the blade. The first rod is approximately 6 cm long and 2 mm in diameter, and the lower rod is broken (Fig. 1, d). There is a short rod inserted into a hole cut through the hilt, parallel to the width and at a right angle to the length (Fig. 1, e).
The iron sword was recovered from the site of Thelunganur (Fig. 2, a), in the Salem district of Tamil Nadu province, by one of the authors (R. R.). It was placed at the bottom of a pit in a ritual context containing an urn enclosed with a capstone. The site lies along the right bank of the River Kaveri, and within the water spread area of Mettur Dam, constructed in 1934. The site is exposed when the water level of the reservoir decreases during summer. The graveyard at Thelunganur covers an area of approximately 80 ha and contains several Iron Age megalithic burials (Fig. 2, b). Megalithic burials in this region consist primarily of subterranean structures, such as pits, urns and chamber tombs, enclosed in an area delineated by cairn or stone circles. The megalithic period in India is closely associated with the emergence of iron metallurgy and black‐and‐red ware, although these two elements had independent origins and spatial distributions. It is difficult to determine when the two elements were culturally integrated with the local megalithic monuments, but in South India they are found in association with both Neolithic–Chalcolithic wares and with inscribed Brahmi script and Roman artefacts. This observation places the time line of their integration somewhere between 1500 and 100 bce . The occurrence of a large number of Neolithic tools and pit burials and the rudimentary nature of the grave goods, however, suggests that the burial site at Thelunganur may well be dated to the sixth century bce or earlier (Rajan 2004; Rajan and Yatheeskumar 2012).
To understand further the date of this object, two radiocarbon measurements on carbon samples extracted directly from two different parts of the sword were made at the University of Arizona's NSF‐Arizona AMS Facility. The 1σ 14C ages, given as years before present (yr bp ) as of 1950, were 3089 ± 40 (AA99857) and 4208 ± 35 (AA104832), which, when calibrated, give calendar dates of approximately the mid‐second and early third millennium bce , respectively (Rajan et al . 2017). Both dates, which are inconsistent and deviate significantly from archaeological contexts, cannot at the moment have any practical value in periodization.
MICROSTRUCTURAL EXAMINATION
The specimens taken from the sword at arrows 1–8 in Fig. 1(a) were mounted, polished and etched with a solution of 2 vols % nitric acid in methanol. They were then examined for microstructures using an optical microscope and a scanning electron microscope (SEM). Hardness measurements were made using a Vickers hardness tester with a load of 500 g applied to a diamond indentation for 15 s. Their approximate carbon levels were inferred from microstructural data and specified according to weight fraction. Other chemical information was obtained using the energy dispersive X‐ray spectrometer (EDS) included with the SEM instrument.
Fig. 3(a–d) are SEM micrographs showing the structures of the specimen taken at arrow 1 of Fig. 1(a): the tip of the sword. The microstructures observed in all the specimens from arrows 1 to 8 were fairly consistent and consisted of the cementite phase precipitated in the form of spherical particles (Fig. 3, a). Exceptions were also found, particularly in the specimen from the tip. The most typical example of such exceptions occurs when the proeutectoid carbide phase, in the form of large‐scale needles or ribbons, was precipitated along the former austenite grain boundaries as well as in the grain interior (Fig. 3, b). Extremely large carbide particles are also present, some of which evidently promoted the formation of micro‐cracks (Fig. 3, c; arrows indicate the location of micro‐cracks). We also conducted a laboratory experiment where a few small fragments from the tip were heated at 1000°C for 10 min and then slowly cooled in an ambient environment. In one specimen, this resulted in the precipitation of large‐scale proeutectoid cementite against the pearlite background consisting of alternating layers of cementite and ferrite (Fig. 3, d). In another specimen, we noted ferrite bands arranged in the pearlite background (Fig. 3, e), indicating that the material under consideration was initially produced in a process involving a solidification reaction (Samuels 1999, 110). Still another specimen was quenched after heating and was found to consist of martensite with a Vickers hardness ranging from 680 to 880. The carbon concentration, inferred from the hardness, is in the range of 0.9–1.3% (Verhoeven 1975), in general agreement with the estimation based on the structure in Fig. 3(d). EDS analyses detected no other elements than iron and carbon.

(a–c) Scanning electron microscope (SEM) micrographs (2000×) showing the varying structures observed in the specimen from near the tip at arrow 1 in Fig. 1(a); (d) SEM micrograph (2000×) showing the structure of the specimen from near the tip following a thermal treatment at 1000°C in an air environment for 10 min before slow cooling to ambient temperatures; and (e) optical micrograph (200×) showing the structure of the specimen from near the tip given the same thermal treatment as that in Fig. 3(d). [Colour figure can be viewed at wileyonlinelibrary.com]
The precipitation of cementite in spherical particles as seen in Fig. 3(a) imparts substantial ductility to high‐carbon steel, allowing it to receive a great deal of mechanical working without suffering brittle fracture. Such a peculiar form of cementite, however, cannot be achieved without a special treatment because austenite generally transforms to pearlite, upon slowly cooling, through the cooperative growth of ferrite and cementite in alternating plates (Fig. 3, d). It has been established in modern metallurgy (Oyama et al . 1984) that the spherical form of cementite precipitation can be obtained by two different methods. The first technique consists of subjecting the high‐carbon steel to prolonged thermal and mechanical treatment at temperatures slightly below the eutectoid isotherm (727°C) in order to transform the cementite in pearlite to spherical particles. In the second method, the steel is first heated in the single‐phase austenite region to dissolve all the carbon and then mechanically worked during cooling to a temperature near 727°C. This treatment promotes proeutectoid cementite to precipitate in the form of spherical particles, with the matrix being transformed to pearlite, during subsequent cooling to room temperature. The specimen is then heated briefly at slightly over 727°C to be austenitized and then slowly cooled in air. During this final cooling stage, spherical cementite precipitates in a reaction termed ‘divorced eutectoid transformation', which suppresses the formation of pearlite.
High‐temperature forging is thus key in handling high‐carbon steel, not only for shaping but also for reducing the carbon content, as either of the above methods could in principle be applied to obtain spherical cementite. In practice, however, the second technique, once established, would be much more effective and practical. In this method, the thermal treatment for inducing the required reaction might be applied only once without mechanical working upon the completion of hot forging. By contrast, the first method requires much time and labour for the prolonged thermal and mechanical treatment at the correct temperature range < 727°C. It is, therefore, inevitable to repeat cycles of heating and forging during this process, and mechanical working involved on the entire specimen would be extremely difficult. This technique is particularly problematic for large‐scale objects, such as the sword under investigation. It is impressive to note that the absolute majority of eutectoid cementite exists in the form of spherical particles in all the specimens examined. This fact points to the application of a treatment leading to a nearly uniform transformation over the entire object, which can readily be achieved using the particular technique. The control of proeutectoid precipitation, however, was not always perfect, as is evident in Fig. 3(b, c). The formation of such large‐scale cementite, which is brittle even at high temperatures, could signify partial failure in the stage of high temperature forging.
All the specimens examined were unusually clean and nearly free of any non‐metallic inclusions. A few specimens, particularly those from near the tip, were found to contain some non‐metallic inclusions in a localized area, one of which is shown at the arrow in Fig. 4(a). The inclusion is seen to consist of a particle embedded in the dark matrix. This could be slag from smelting which remained entrapped in the metal matrix. EDS analysis on the particle, however, shows that its primary constituents are calcium and titanium, both probably in the form of oxide (Fig. 4, b). As such, it cannot be a particle of iron ore remaining unreduced from smelting nor can it be a particle precipitated from a reaction involving iron‐bearing materials in a solid or a liquid state. The particle may, therefore, have been incorporated during the smelting or smithy process. EDS analyses on the matrix surrounding the particle showed that its iron content was greatly increased while the calcium and titanium levels were significantly lowered. EDS analyses, performed on other such particles, produced similar results.
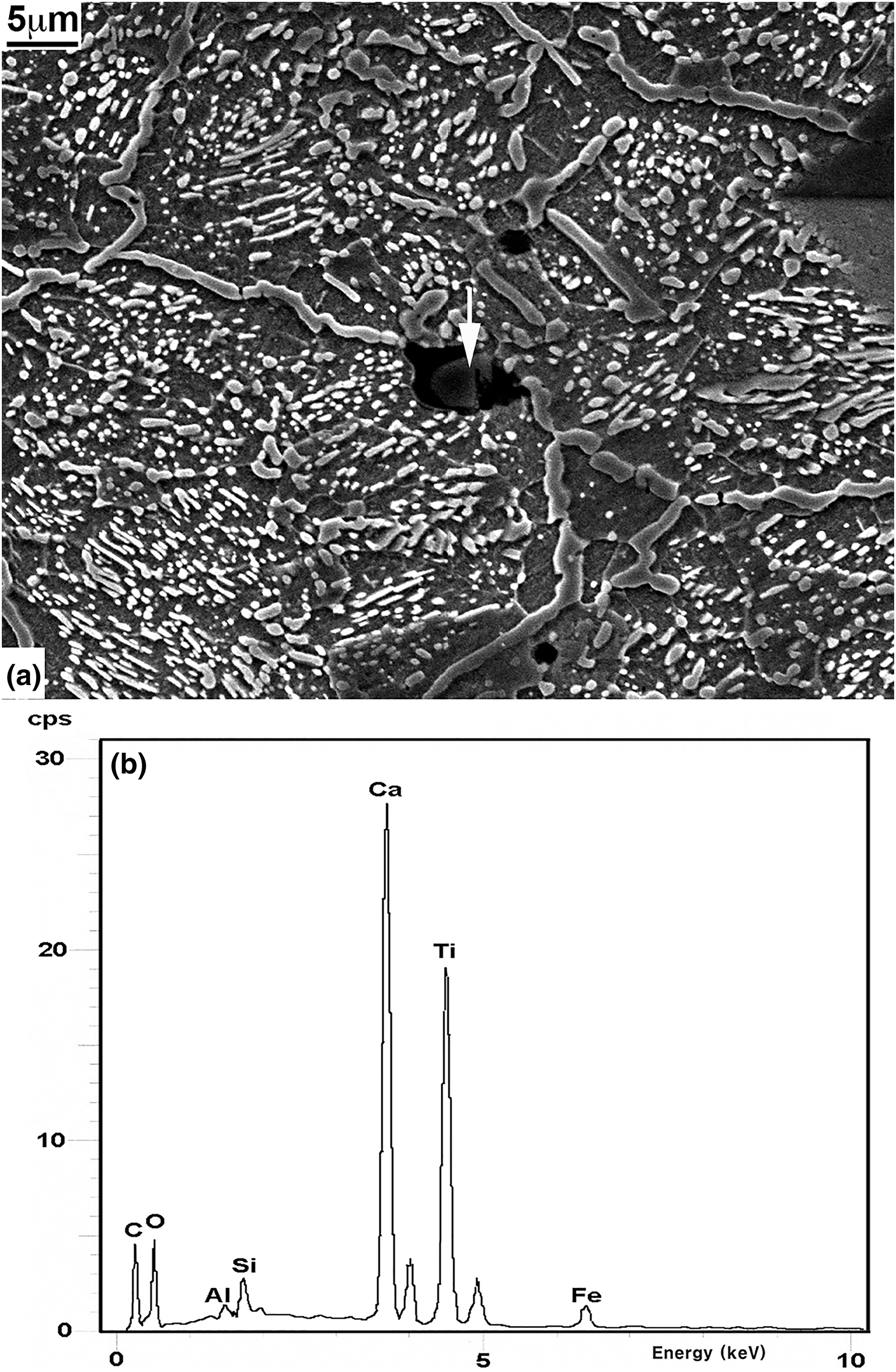
(a) Scanning electron microscope (SEM) micrograph (2000×) showing the structure of the specimen from near the tip at arrow 1 in Fig. 1(a); and (b) energy dispersive X‐ray spectrometer (EDS) spectrum taken from the arrow shown in Fig. 4(a).
Fig. 5(a), an optical micrograph covering the entire cross‐section of the specimen from the tip, reveals a laminated structure consisting of layers running horizontally. The presence of these layers was found to create colorful patterns on the polished and etched surface, which were clearly visible to the naked eye. The rhombuses in the micrograph represent impressions made during the hardness measurements, which correspond to Vickers hardness of 250–290, with the dark area producing the higher values. Fig. 5(b, c), SEM micrographs magnifying the areas near arrows A and B in Fig. 5(a), respectively, provide detailed views of the morphological characteristics of the two areas. Their background structure is similar and consists of fine carbide particles or filaments in the dark ferrite matrix. A major difference, however, is found in the relatively large‐scale cementite phase, which is in the form of plates in Fig. 5(c) as opposed to the spherical form in Fig. 5(b). Evidently, the difference in shape and size of the iron carbide phase is responsible for the layered structures developed in Fig. 5(a), and helps create the visible effect.
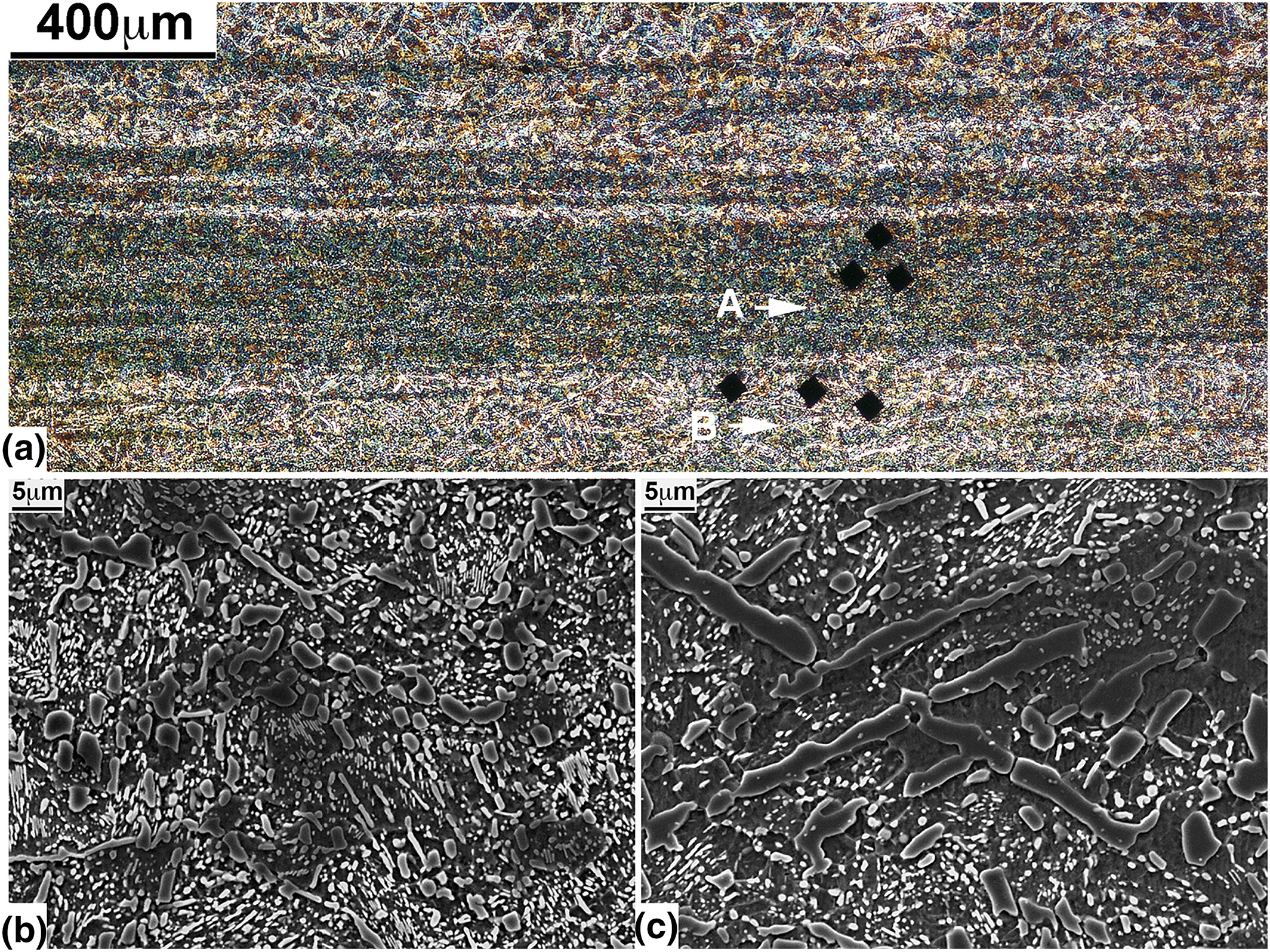
Micrographs showing the structure of the specimen from near the tip at arrow 1 in Fig. 1(a): (a) optical micrograph (50×) showing the presence of microscopic layers producing a colorful pattern visible on the polished and etched surface; (b, c) scanning electron microscope (SEM) micrographs (2000×) enlarging the area marked by arrows 1 and 2 in Fig. 5(a), respectively. [Colour figure can be viewed at wileyonlinelibrary.com]
DISCUSSION
The microstructural data presented above show that the sword under investigation was forged out of high‐carbon steel whose carbon content ranges from approximately 0.9% to 1.3%. The use of such high‐carbon steel in antiquity raises important questions about how it was made and processed, despite its brittleness, into such an important functional object as a sword. Another issue associated with these questions involves the fabrication of certain swords famed for the surface pattern visible on their blades. It has been well established that they were forged from special crucible steel by applying highly sophisticated thermal and mechanical treatments (Verhoeven et al . 1996), and it is tempting to presume that similar materials and processing technologies were applied to the sword examined. The fabrication of such swords, however, was a relatively recent practice, while the information currently available on the crucible process places its origin within a few centuries of the beginning of the first millennium ce (Bronson 1986; Craddock 1998; Park and Shinde 2013b). Unfortunately, the radiocarbon measurements on carbon samples directly extracted from two different sword specimens produced results that are not consistent. Moreover, the radiocarbon ages depart considerably from, and are much older than, other lines of relative chronological evidence, which placed the date in the mid‐first millennium bce or earlier. When relying on its recovery context (Rajan 2004; Rajan and Yatheeskumar 2012), the object may be considered early archaeological evidence suggesting the production of high‐carbon materials and it provides a unique window into the technologies employed in Indian antiquity for the production and processing of such high‐carbon steel.
The raw material used in the sword is clearly distinguished from low‐carbon iron from bloomery smelting, which had long formed the foundation of traditional Indian iron industry (Park and Shinde 2013a). Its carbon content is very high and fairly uniform over the entire object. There is some variation in microscopic‐scale carbon distribution, but there is no noticeable difference observed in the overall carbon concentration between specimens. In addition, the specimens examined are unusually clean and nearly free of any kind of non‐metallic inclusions. The few non‐metallic inclusions that do exist, however, are distinctly different in chemical composition from those commonly incorporated in bloomery iron. It is not impossible to produce steel by carburizing bloomery iron in the solid state. In many instances, however, superior material properties can readily be obtained at relatively low carbon levels and there is no point wasting time and labour to use this technique for making such high‐carbon steel, which is notorious for its brittleness. In contrast, the process involving high‐carbon iron in a fully molten state can allow for all the features found in the specimens examined, that is, a high carbon content, a uniform carbon distribution and lack of slag inclusions. Moreover, the ferrite bands, which developed in one of the specimens treated in the laboratory experiments, confirmed that the raw material was formed in the liquid state.
It is important to note, however, that high‐carbon steel could directly be obtained from the smelting process, as pointed out by Juleff (1996) and exemplified in the Japanese Tatara process and its African counterparts (Rostoker and Bronson 1990) as well as in some African iron smelting in natural draft furnaces (Killick 1991). The Tatara process produces cast iron, steel and low‐carbon iron in a single operation (Tylecote 1992; Park 2004). Apart from the cast iron, the Tatara product forms a bloom or an ingot where the carbon concentration varies to cover the entire range from almost pure iron to ultra‐high‐carbon steel. Despite its being produced in the solid state, the Tatara ingot is relatively free of non‐metallic inclusions as compared with other bloomery products. In addition, it contains a portion where the carbon level is comparable with that observed in Indian crucible steel. Its greatly varying carbon content, however, is an aspect absent in crucible steel, which makes it difficult, if not impossible, to achieve the homogeneous microstructure as observed in the sword specimens under consideration. The use of a raw material with such a variable carbon level often requires a special fabrication process where its carbon concentration is homogenized while it is being made into a finished functional product. The thermo‐mechanical treatments applied in this case focuses primarily on the control of carbon distribution without paying much attention to the control of cementite morphologies (Wayman 1998; Park 2004).
Crucible or other high‐carbon steel, owing to its high carbon content, is generally brittle and prone to brittle fracture upon impact in fabrication or service. This brittleness arises primarily from the precipitation of proeutectoid cementite, which forms a brittle carbide network along prior austenite grain boundaries. The formation of such proeutectoid cementite in high‐carbon steel is unavoidable with slow cooling. High‐carbon steel is of little value, therefore, unless a special technology is developed to make its microstructure free of such a harmful constituent. This explains why ultra‐high‐carbon steel was not a material of interest for the modern iron industry until a thermomechanical treatment to induce the divorced eutectoid transformation was discovered and applied to control the carbide morphology effectively. This phenomenon, reported early in the 20th century ce , has been extensively studied (Oyama et al . 1984; Sherby et al . 1985; Syn et al . 1994; Verhoeven and Gibson 1998) and applied as an economical technique for the processing of high‐carbon steel. Surprisingly, evidence of the application of this modern technique is confirmed in the ancient blade under investigation.
In successful divorced eutectoid transformation treatments, which require mechanical working, both the proeutectoid and eutectoid transformations are controlled to promote the precipitation of cementite in the form of fine particles, instead of the plate form found in pearlite. Typical products of this unique transformation technique are seen in Fig. 3(a), which contains no notable proeutectoid cementite precipitated to form carbide networks at austenite grain boundaries. Fig. 3(a) consists primarily of fine carbide particles in the ferrite matrix. With this particular microstructure, high‐carbon steel can serve as a unique functional material guaranteeing both strength and ductility to such an extent not obtainable otherwise in antiquity. The structures in Fig. 3(b, c), where some cementite created networks or existed as large‐scale particles, show that the morphological control was not perfect. The harmful effect of this imperfection is evident in Fig. 3(c) where cracks were created within some of the coarse carbide particles.
It has been reported that the particular transformation technique, combined with mechanical working, was employed to reconstruct the technology for making Damascus swords (Mough 1982; Verhoeven et al . 1996). Verhoeven et al . (1996) showed that certain alloying elements inadvertently introduced in small amounts played a key role in the formation of the damask pattern. These elements have a tendency to segregate and promote band or layered structures by inducing selective coarsening of carbide particles in the layers in which they are enriched. He used the term ‘genuine Damascus blades' only for those made from crucible steel produced in southern India, suggesting that the specific elements were unique to wootz but not general to all crucible steels. The concentration of the specific elements in all the specimens examined here, however, was below the detection limit of the EDS instrument. Furthermore, with the given specimens, no other technique can be employed to obtain such accurate chemical information. Nevertheless, certain laminated patterns arising from variations in carbide morphology are visible in the specimens. These patterns may not be of the same character as those found in genuine Damascus swords, but they may not have gone unnoticed. Once recognized, the patterns may have served as a force driving the development of various surface patterns including the damask.
SUMMARY AND CONCLUSIONS
The metallographic examination of a double‐edged iron sword recovered from a ritual context of an Iron Age megalithic site at Thelunganur in Tamil Nadu, India, showed that it was forged out of high‐carbon steel with a carbon content of 0.9–1.3%. The specimens examined were clean and free of any notable non‐metallic inclusions. Their microstructures were fairly uniform and consisted primarily of fine spherical cementite particles in the ferrite background. The high carbon content, absence of slag inclusions and uniform microstructure—rarely obtained in bloomery smelting—are features characteristic of crucible steel produced from a fully molten state. In addition, the formation of ferrite bands in one specimen given the proper thermal treatment confirms that the material examined was the product of a solidification reaction. It may therefore be suggested that the blade under investigation could possibly be one of the earliest examples of a crucible steel product.
More importantly, the carbide phase precipitated in the form of fine spherical particles provides clear evidence of special thermomechanical treatments applied in the processing of such a brittle material for fabrication. The treatments likely involved the divorced eutectoid transformation technique, which was rediscovered in the 20th century ce for the effective exploitation of high‐carbon steel. The shape and size of cementite were not perfectly uniform, which in some cases caused a laminated structure to develop in the microstructure, creating colorful patterns visible on the polished and etched surface. This pattern, once noticed, may have inspired the later development of varying patterns leading to the famous damask.
The above results show that technologies needed to facilitate both the production and the processing of crucible or other high‐carbon steel were largely established in the Thelunganur region of Tamil Nadu by the time the sword was made, approximately around the mid‐first millennium bce . The application of such technologies, however, was apparently not always perfect and might often end up with a tragic and premature failure of crucible steel products.
ACKNOWLEDGEMENTS
This work would not have been possible without the kind support from the people at Pondicherry University who showed hospitality to one of the authors (J.S.P.) and his wife when they visited to take samples for examination. The authors thank Mr Jay Andrew Stephens for linguistic assistance with this manuscript. This work, finished while J.S.P. was a visiting scholar at the School of Anthropology, University of Arizona, was financially supported by the National Research Foundation of Korea (grant number NRF‐2017R1A2B4002082).
REFERENCES
 onlinelibrary.wiley.com
onlinelibrary.wiley.com
I usually only like to post bits and pieces of a source, but this source is WAAAAAAYYY to good of a gem to be lost.

CC
J.‐S. Park- Department of Materials Science and Engineering, Hongik University, 2639 Sejong‐ro, Jochiwon, Sejong, 30016 South Korea
K. Rajan - Department of History, Pondicherry University, Puducherry, Tamil Nadu, 605014 India
R. Ramesh - Archaeological Survey of India, Chennai Circle, Tamil Nadu, 600 009 India
An iron sword from an Iron Age megalithic burial at Thelunganur in Tamil Nadu, India, was examined using metallographic techniques. The sword was made of ultra‐high‐carbon steel with a fairly uniform microstructure consisting primarily of fine cementite particles in a ferrite background free of notable non‐metallic inclusions. The morphological control, however, was not perfect and frequently allowed cementite to precipitate in the form of a network along austenite grain boundaries. It was also observed that carbide particles of varying size and shape often caused microscopic layers to develop, forming a visible pattern to the naked eye on the polished and etched surface of the iron sword. This pattern likely inspired the later development of various surface markings such as the damask. This paper presents a detailed account of the analytical data to show that the iron sword under consideration was an early example of high‐carbon steel employed in the manufacture of a functional object where the divorced eutectoid transformation technique, rediscovered recently, was used for the control of cementite morphology. It is also proposed that technologies for making and handling high‐carbon steel were in existence at a much earlier date than previously supposed.
INTRODUCTION
Carbon concentration and its distribution pattern constitute two key factors that determine the functional properties of an iron sword, which can be optimized by taking the balance between strength and ductility. The selection of raw materials therefore plays an important role in the establishment of a particular sword‐making technology. If high‐carbon steel were used to take advantage of its high strength, it would be necessary to improve the low‐impact resistance arising from its high carbon concentration.
India was famed for the early production of a special high‐carbon material termed crucible steel. In his survey research on traditional Indian crucible steel, wootz, Bronson (1986) concluded that it was produced by a wide range of processes, not only just as super steel but also as a material for common use. Despite its fame gained as the raw material for Damascus blades, Bronson noted that crucible steel was not a material of choice among warriors, primarily due to its brittleness. This brittleness arises from its high carbon concentration, which is generally > 1%. (Chemical compositions in this paper are based on weight fraction.) Such high‐carbon steel contains a substantial amount of iron carbide (Fe3C), termed cementite, which is brittle even at temperatures up to its melting point. This brittleness poses serious difficulties in fabrication or service and cannot be overcome without a high level of technological sophistication.
Raw materials similar in carbon levels to Indian crucible steel were produced elsewhere in the world using a variety of techniques, most of which involved the use of cast iron in one way or another. Such high‐carbon materials were then employed as intermediaries to be made into finished items with processes that caused a substantial reduction of their carbon level (Park 2004, 2005, 2008). Evidence was found, however, that the technology for processing high‐carbon steel in India was rather unique and focused on controlling the shape, size and distribution of the cementite phase (Park and Shinde 2013b). In a certain sense, therefore, the discovery of proper techniques for handling such high‐carbon materials signifies the beginning of crucible or other high‐carbon technologies, and Indian ironworkers should thus also be recognized for their in vention of such processing methods.
Bronson (1986) traced the beginning of Indian crucible technology to the second century ce , at the earliest, by refuting the prevailing hypotheses of earlier dates based on certain classical sources of the 5th‐century bce onwards. He also rejected Hadfield's interpretation that some of the iron objects excavated from the Sirkap site at Taxila, Pakistan, were made of crucible steel (Marshall 1945). In a subsequent review article drawing on an extended body of literary and archaeological evidence, Craddock (1998) reinforced most of Bronson's conclusions. Craddock, however, regarded the Taxila iron objects examined by Hadfield as an example of crucible steel, and suggested that crucible processes were practised by the first centuries of the first millennium ce . On the other hand, recent studies (Srinivasan 2007; Srinivasan et al . 2009), based on the investigation of crucible fragments and metal particles recovered from ancient sites in Tamil Nadu (Rajan 1997), proposed that the crucible process was likely practised as early as the mid‐first millennium bce .
Despite substantial debate over the origin and production of Indian crucible steel, little is known of thermomechanical treatments developed for the fabrication of such high‐carbon materials into finished products. Much work (Verhoeven and Peterson 1992; Verhoeven et al . 1996) has been done on Damascus swords to understand the mechanisms responsible for the emergence of their unique surface patterns. However, the information from such research cannot be used to infer technologies practised in Indian antiquity. In this respect, the recent work of Park and Shinde (2013b) is significant as it reported on a small piece of unprocessed high‐carbon steel and small finished items evidently made from such a high‐carbon material. In addition, they produced radiocarbon data placing the date of manufacture to the last few centuries of the first millennium bce . The objects these authors examined, however, were ordinary items weighing approximately 10 g, or less. Their data, therefore, cannot be extended to address the technological and chronological aspects associated with the use of high‐carbon steel in the manufacture of important functional items, such as swords. Similarly, the analysis of a Sasanian sword dating to the sixth to seventh centuries ce (Lang et al . 1998), one of the earliest examples of such products thus far examined, is also far from sufficient.
A unique opportunity to resolve this lack of information is provided by an iron sword (Fig. 1) recently recovered from an Iron Age megalithic burial site at Thelunganur in the Salem district of Tamil Nadu, India (Fig. 2). The site can be dated to the sixth century bce or earlier based on typological grounds (Rajan 2004; Rajan and Yatheeskumar 2012), indicating that the sword could be an early archaeological example of a high‐carbon material successfully processed into a finished product. The results presented below are expected to shed a new light upon the discussion of Indian high‐carbon steel in terms of its technology and chronology.
General appearance of the double‐edged iron sword under investigation: (a) general appearance, where arrows locate the spots from which specimens were taken for metallographic examination; (b, c) magnified view of the tip and the body, respectively; and (d, e) magnified views of the hilt. [Colour figure can be viewed at wileyonlinelibrary.com]
COMMENTS ON ARTEFACT AND SITE
Fig. 1(a) shows the general appearance of the sword, with the arrows indicating the spots from which tiny test specimens were taken for examination. The sword is double edged, approximately 88 cm long and 930 g in weight. The blade is around 4.7 cm wide near arrow 4 and becomes narrower in both directions toward the hilt and the tip. It is 3.7 cm wide where the hilt begins. Fig. 1(b, c) show two parallel grooves, approximately 5 mm apart, incised along the midrib. They start approximately 10 cm from the hilt and run toward the tip until they are barely visible against the corroded surface near the tip. The midrib of the blade is around 3.4 mm thick near arrow 3 and 7.7 mm thick between arrows 5 and 6. The tip near arrow 1 is ≤ 1 mm in thickness, indicating that it was subjected to significantly more plastic flow than the other parts. The hilt of the blade was presumably designed for hafting (Fig. 1, d, e), and there are two rods extending from the upper edge of the blade. The first rod is approximately 6 cm long and 2 mm in diameter, and the lower rod is broken (Fig. 1, d). There is a short rod inserted into a hole cut through the hilt, parallel to the width and at a right angle to the length (Fig. 1, e).
The iron sword was recovered from the site of Thelunganur (Fig. 2, a), in the Salem district of Tamil Nadu province, by one of the authors (R. R.). It was placed at the bottom of a pit in a ritual context containing an urn enclosed with a capstone. The site lies along the right bank of the River Kaveri, and within the water spread area of Mettur Dam, constructed in 1934. The site is exposed when the water level of the reservoir decreases during summer. The graveyard at Thelunganur covers an area of approximately 80 ha and contains several Iron Age megalithic burials (Fig. 2, b). Megalithic burials in this region consist primarily of subterranean structures, such as pits, urns and chamber tombs, enclosed in an area delineated by cairn or stone circles. The megalithic period in India is closely associated with the emergence of iron metallurgy and black‐and‐red ware, although these two elements had independent origins and spatial distributions. It is difficult to determine when the two elements were culturally integrated with the local megalithic monuments, but in South India they are found in association with both Neolithic–Chalcolithic wares and with inscribed Brahmi script and Roman artefacts. This observation places the time line of their integration somewhere between 1500 and 100 bce . The occurrence of a large number of Neolithic tools and pit burials and the rudimentary nature of the grave goods, however, suggests that the burial site at Thelunganur may well be dated to the sixth century bce or earlier (Rajan 2004; Rajan and Yatheeskumar 2012).
To understand further the date of this object, two radiocarbon measurements on carbon samples extracted directly from two different parts of the sword were made at the University of Arizona's NSF‐Arizona AMS Facility. The 1σ 14C ages, given as years before present (yr bp ) as of 1950, were 3089 ± 40 (AA99857) and 4208 ± 35 (AA104832), which, when calibrated, give calendar dates of approximately the mid‐second and early third millennium bce , respectively (Rajan et al . 2017). Both dates, which are inconsistent and deviate significantly from archaeological contexts, cannot at the moment have any practical value in periodization.
MICROSTRUCTURAL EXAMINATION
The specimens taken from the sword at arrows 1–8 in Fig. 1(a) were mounted, polished and etched with a solution of 2 vols % nitric acid in methanol. They were then examined for microstructures using an optical microscope and a scanning electron microscope (SEM). Hardness measurements were made using a Vickers hardness tester with a load of 500 g applied to a diamond indentation for 15 s. Their approximate carbon levels were inferred from microstructural data and specified according to weight fraction. Other chemical information was obtained using the energy dispersive X‐ray spectrometer (EDS) included with the SEM instrument.
Fig. 3(a–d) are SEM micrographs showing the structures of the specimen taken at arrow 1 of Fig. 1(a): the tip of the sword. The microstructures observed in all the specimens from arrows 1 to 8 were fairly consistent and consisted of the cementite phase precipitated in the form of spherical particles (Fig. 3, a). Exceptions were also found, particularly in the specimen from the tip. The most typical example of such exceptions occurs when the proeutectoid carbide phase, in the form of large‐scale needles or ribbons, was precipitated along the former austenite grain boundaries as well as in the grain interior (Fig. 3, b). Extremely large carbide particles are also present, some of which evidently promoted the formation of micro‐cracks (Fig. 3, c; arrows indicate the location of micro‐cracks). We also conducted a laboratory experiment where a few small fragments from the tip were heated at 1000°C for 10 min and then slowly cooled in an ambient environment. In one specimen, this resulted in the precipitation of large‐scale proeutectoid cementite against the pearlite background consisting of alternating layers of cementite and ferrite (Fig. 3, d). In another specimen, we noted ferrite bands arranged in the pearlite background (Fig. 3, e), indicating that the material under consideration was initially produced in a process involving a solidification reaction (Samuels 1999, 110). Still another specimen was quenched after heating and was found to consist of martensite with a Vickers hardness ranging from 680 to 880. The carbon concentration, inferred from the hardness, is in the range of 0.9–1.3% (Verhoeven 1975), in general agreement with the estimation based on the structure in Fig. 3(d). EDS analyses detected no other elements than iron and carbon.
(a–c) Scanning electron microscope (SEM) micrographs (2000×) showing the varying structures observed in the specimen from near the tip at arrow 1 in Fig. 1(a); (d) SEM micrograph (2000×) showing the structure of the specimen from near the tip following a thermal treatment at 1000°C in an air environment for 10 min before slow cooling to ambient temperatures; and (e) optical micrograph (200×) showing the structure of the specimen from near the tip given the same thermal treatment as that in Fig. 3(d). [Colour figure can be viewed at wileyonlinelibrary.com]
The precipitation of cementite in spherical particles as seen in Fig. 3(a) imparts substantial ductility to high‐carbon steel, allowing it to receive a great deal of mechanical working without suffering brittle fracture. Such a peculiar form of cementite, however, cannot be achieved without a special treatment because austenite generally transforms to pearlite, upon slowly cooling, through the cooperative growth of ferrite and cementite in alternating plates (Fig. 3, d). It has been established in modern metallurgy (Oyama et al . 1984) that the spherical form of cementite precipitation can be obtained by two different methods. The first technique consists of subjecting the high‐carbon steel to prolonged thermal and mechanical treatment at temperatures slightly below the eutectoid isotherm (727°C) in order to transform the cementite in pearlite to spherical particles. In the second method, the steel is first heated in the single‐phase austenite region to dissolve all the carbon and then mechanically worked during cooling to a temperature near 727°C. This treatment promotes proeutectoid cementite to precipitate in the form of spherical particles, with the matrix being transformed to pearlite, during subsequent cooling to room temperature. The specimen is then heated briefly at slightly over 727°C to be austenitized and then slowly cooled in air. During this final cooling stage, spherical cementite precipitates in a reaction termed ‘divorced eutectoid transformation', which suppresses the formation of pearlite.
High‐temperature forging is thus key in handling high‐carbon steel, not only for shaping but also for reducing the carbon content, as either of the above methods could in principle be applied to obtain spherical cementite. In practice, however, the second technique, once established, would be much more effective and practical. In this method, the thermal treatment for inducing the required reaction might be applied only once without mechanical working upon the completion of hot forging. By contrast, the first method requires much time and labour for the prolonged thermal and mechanical treatment at the correct temperature range < 727°C. It is, therefore, inevitable to repeat cycles of heating and forging during this process, and mechanical working involved on the entire specimen would be extremely difficult. This technique is particularly problematic for large‐scale objects, such as the sword under investigation. It is impressive to note that the absolute majority of eutectoid cementite exists in the form of spherical particles in all the specimens examined. This fact points to the application of a treatment leading to a nearly uniform transformation over the entire object, which can readily be achieved using the particular technique. The control of proeutectoid precipitation, however, was not always perfect, as is evident in Fig. 3(b, c). The formation of such large‐scale cementite, which is brittle even at high temperatures, could signify partial failure in the stage of high temperature forging.
All the specimens examined were unusually clean and nearly free of any non‐metallic inclusions. A few specimens, particularly those from near the tip, were found to contain some non‐metallic inclusions in a localized area, one of which is shown at the arrow in Fig. 4(a). The inclusion is seen to consist of a particle embedded in the dark matrix. This could be slag from smelting which remained entrapped in the metal matrix. EDS analysis on the particle, however, shows that its primary constituents are calcium and titanium, both probably in the form of oxide (Fig. 4, b). As such, it cannot be a particle of iron ore remaining unreduced from smelting nor can it be a particle precipitated from a reaction involving iron‐bearing materials in a solid or a liquid state. The particle may, therefore, have been incorporated during the smelting or smithy process. EDS analyses on the matrix surrounding the particle showed that its iron content was greatly increased while the calcium and titanium levels were significantly lowered. EDS analyses, performed on other such particles, produced similar results.
(a) Scanning electron microscope (SEM) micrograph (2000×) showing the structure of the specimen from near the tip at arrow 1 in Fig. 1(a); and (b) energy dispersive X‐ray spectrometer (EDS) spectrum taken from the arrow shown in Fig. 4(a).
Fig. 5(a), an optical micrograph covering the entire cross‐section of the specimen from the tip, reveals a laminated structure consisting of layers running horizontally. The presence of these layers was found to create colorful patterns on the polished and etched surface, which were clearly visible to the naked eye. The rhombuses in the micrograph represent impressions made during the hardness measurements, which correspond to Vickers hardness of 250–290, with the dark area producing the higher values. Fig. 5(b, c), SEM micrographs magnifying the areas near arrows A and B in Fig. 5(a), respectively, provide detailed views of the morphological characteristics of the two areas. Their background structure is similar and consists of fine carbide particles or filaments in the dark ferrite matrix. A major difference, however, is found in the relatively large‐scale cementite phase, which is in the form of plates in Fig. 5(c) as opposed to the spherical form in Fig. 5(b). Evidently, the difference in shape and size of the iron carbide phase is responsible for the layered structures developed in Fig. 5(a), and helps create the visible effect.
Micrographs showing the structure of the specimen from near the tip at arrow 1 in Fig. 1(a): (a) optical micrograph (50×) showing the presence of microscopic layers producing a colorful pattern visible on the polished and etched surface; (b, c) scanning electron microscope (SEM) micrographs (2000×) enlarging the area marked by arrows 1 and 2 in Fig. 5(a), respectively. [Colour figure can be viewed at wileyonlinelibrary.com]
DISCUSSION
The microstructural data presented above show that the sword under investigation was forged out of high‐carbon steel whose carbon content ranges from approximately 0.9% to 1.3%. The use of such high‐carbon steel in antiquity raises important questions about how it was made and processed, despite its brittleness, into such an important functional object as a sword. Another issue associated with these questions involves the fabrication of certain swords famed for the surface pattern visible on their blades. It has been well established that they were forged from special crucible steel by applying highly sophisticated thermal and mechanical treatments (Verhoeven et al . 1996), and it is tempting to presume that similar materials and processing technologies were applied to the sword examined. The fabrication of such swords, however, was a relatively recent practice, while the information currently available on the crucible process places its origin within a few centuries of the beginning of the first millennium ce (Bronson 1986; Craddock 1998; Park and Shinde 2013b). Unfortunately, the radiocarbon measurements on carbon samples directly extracted from two different sword specimens produced results that are not consistent. Moreover, the radiocarbon ages depart considerably from, and are much older than, other lines of relative chronological evidence, which placed the date in the mid‐first millennium bce or earlier. When relying on its recovery context (Rajan 2004; Rajan and Yatheeskumar 2012), the object may be considered early archaeological evidence suggesting the production of high‐carbon materials and it provides a unique window into the technologies employed in Indian antiquity for the production and processing of such high‐carbon steel.
The raw material used in the sword is clearly distinguished from low‐carbon iron from bloomery smelting, which had long formed the foundation of traditional Indian iron industry (Park and Shinde 2013a). Its carbon content is very high and fairly uniform over the entire object. There is some variation in microscopic‐scale carbon distribution, but there is no noticeable difference observed in the overall carbon concentration between specimens. In addition, the specimens examined are unusually clean and nearly free of any kind of non‐metallic inclusions. The few non‐metallic inclusions that do exist, however, are distinctly different in chemical composition from those commonly incorporated in bloomery iron. It is not impossible to produce steel by carburizing bloomery iron in the solid state. In many instances, however, superior material properties can readily be obtained at relatively low carbon levels and there is no point wasting time and labour to use this technique for making such high‐carbon steel, which is notorious for its brittleness. In contrast, the process involving high‐carbon iron in a fully molten state can allow for all the features found in the specimens examined, that is, a high carbon content, a uniform carbon distribution and lack of slag inclusions. Moreover, the ferrite bands, which developed in one of the specimens treated in the laboratory experiments, confirmed that the raw material was formed in the liquid state.
It is important to note, however, that high‐carbon steel could directly be obtained from the smelting process, as pointed out by Juleff (1996) and exemplified in the Japanese Tatara process and its African counterparts (Rostoker and Bronson 1990) as well as in some African iron smelting in natural draft furnaces (Killick 1991). The Tatara process produces cast iron, steel and low‐carbon iron in a single operation (Tylecote 1992; Park 2004). Apart from the cast iron, the Tatara product forms a bloom or an ingot where the carbon concentration varies to cover the entire range from almost pure iron to ultra‐high‐carbon steel. Despite its being produced in the solid state, the Tatara ingot is relatively free of non‐metallic inclusions as compared with other bloomery products. In addition, it contains a portion where the carbon level is comparable with that observed in Indian crucible steel. Its greatly varying carbon content, however, is an aspect absent in crucible steel, which makes it difficult, if not impossible, to achieve the homogeneous microstructure as observed in the sword specimens under consideration. The use of a raw material with such a variable carbon level often requires a special fabrication process where its carbon concentration is homogenized while it is being made into a finished functional product. The thermo‐mechanical treatments applied in this case focuses primarily on the control of carbon distribution without paying much attention to the control of cementite morphologies (Wayman 1998; Park 2004).
Crucible or other high‐carbon steel, owing to its high carbon content, is generally brittle and prone to brittle fracture upon impact in fabrication or service. This brittleness arises primarily from the precipitation of proeutectoid cementite, which forms a brittle carbide network along prior austenite grain boundaries. The formation of such proeutectoid cementite in high‐carbon steel is unavoidable with slow cooling. High‐carbon steel is of little value, therefore, unless a special technology is developed to make its microstructure free of such a harmful constituent. This explains why ultra‐high‐carbon steel was not a material of interest for the modern iron industry until a thermomechanical treatment to induce the divorced eutectoid transformation was discovered and applied to control the carbide morphology effectively. This phenomenon, reported early in the 20th century ce , has been extensively studied (Oyama et al . 1984; Sherby et al . 1985; Syn et al . 1994; Verhoeven and Gibson 1998) and applied as an economical technique for the processing of high‐carbon steel. Surprisingly, evidence of the application of this modern technique is confirmed in the ancient blade under investigation.
In successful divorced eutectoid transformation treatments, which require mechanical working, both the proeutectoid and eutectoid transformations are controlled to promote the precipitation of cementite in the form of fine particles, instead of the plate form found in pearlite. Typical products of this unique transformation technique are seen in Fig. 3(a), which contains no notable proeutectoid cementite precipitated to form carbide networks at austenite grain boundaries. Fig. 3(a) consists primarily of fine carbide particles in the ferrite matrix. With this particular microstructure, high‐carbon steel can serve as a unique functional material guaranteeing both strength and ductility to such an extent not obtainable otherwise in antiquity. The structures in Fig. 3(b, c), where some cementite created networks or existed as large‐scale particles, show that the morphological control was not perfect. The harmful effect of this imperfection is evident in Fig. 3(c) where cracks were created within some of the coarse carbide particles.
It has been reported that the particular transformation technique, combined with mechanical working, was employed to reconstruct the technology for making Damascus swords (Mough 1982; Verhoeven et al . 1996). Verhoeven et al . (1996) showed that certain alloying elements inadvertently introduced in small amounts played a key role in the formation of the damask pattern. These elements have a tendency to segregate and promote band or layered structures by inducing selective coarsening of carbide particles in the layers in which they are enriched. He used the term ‘genuine Damascus blades' only for those made from crucible steel produced in southern India, suggesting that the specific elements were unique to wootz but not general to all crucible steels. The concentration of the specific elements in all the specimens examined here, however, was below the detection limit of the EDS instrument. Furthermore, with the given specimens, no other technique can be employed to obtain such accurate chemical information. Nevertheless, certain laminated patterns arising from variations in carbide morphology are visible in the specimens. These patterns may not be of the same character as those found in genuine Damascus swords, but they may not have gone unnoticed. Once recognized, the patterns may have served as a force driving the development of various surface patterns including the damask.
SUMMARY AND CONCLUSIONS
The metallographic examination of a double‐edged iron sword recovered from a ritual context of an Iron Age megalithic site at Thelunganur in Tamil Nadu, India, showed that it was forged out of high‐carbon steel with a carbon content of 0.9–1.3%. The specimens examined were clean and free of any notable non‐metallic inclusions. Their microstructures were fairly uniform and consisted primarily of fine spherical cementite particles in the ferrite background. The high carbon content, absence of slag inclusions and uniform microstructure—rarely obtained in bloomery smelting—are features characteristic of crucible steel produced from a fully molten state. In addition, the formation of ferrite bands in one specimen given the proper thermal treatment confirms that the material examined was the product of a solidification reaction. It may therefore be suggested that the blade under investigation could possibly be one of the earliest examples of a crucible steel product.
More importantly, the carbide phase precipitated in the form of fine spherical particles provides clear evidence of special thermomechanical treatments applied in the processing of such a brittle material for fabrication. The treatments likely involved the divorced eutectoid transformation technique, which was rediscovered in the 20th century ce for the effective exploitation of high‐carbon steel. The shape and size of cementite were not perfectly uniform, which in some cases caused a laminated structure to develop in the microstructure, creating colorful patterns visible on the polished and etched surface. This pattern, once noticed, may have inspired the later development of varying patterns leading to the famous damask.
The above results show that technologies needed to facilitate both the production and the processing of crucible or other high‐carbon steel were largely established in the Thelunganur region of Tamil Nadu by the time the sword was made, approximately around the mid‐first millennium bce . The application of such technologies, however, was apparently not always perfect and might often end up with a tragic and premature failure of crucible steel products.
ACKNOWLEDGEMENTS
This work would not have been possible without the kind support from the people at Pondicherry University who showed hospitality to one of the authors (J.S.P.) and his wife when they visited to take samples for examination. The authors thank Mr Jay Andrew Stephens for linguistic assistance with this manuscript. This work, finished while J.S.P. was a visiting scholar at the School of Anthropology, University of Arizona, was financially supported by the National Research Foundation of Korea (grant number NRF‐2017R1A2B4002082).
REFERENCES
Error - Cookies Turned Off
I usually only like to post bits and pieces of a source, but this source is WAAAAAAYYY to good of a gem to be lost.
CC
J.‐S. Park- Department of Materials Science and Engineering, Hongik University, 2639 Sejong‐ro, Jochiwon, Sejong, 30016 South Korea
K. Rajan - Department of History, Pondicherry University, Puducherry, Tamil Nadu, 605014 India
R. Ramesh - Archaeological Survey of India, Chennai Circle, Tamil Nadu, 600 009 India
Last edited:
